When you think about groundbreaking chemistry concepts, one name pops up almost immediately: Arrhenius. This dude wasn’t just some random scientist chilling in a lab; he was a game-changer. Svante Arrhenius, the mind behind the acid-base theory, literally transformed how we understand chemical reactions. His work? A foundation for modern chemistry as we know it. But let’s not kid ourselves, there’s so much more to this guy than just his scientific contributions. Let’s dive deep into his world, shall we?
Arrhenius wasn’t always the celebrated genius we know today. Back in his day, he faced some serious pushback from the scientific community. Can you believe that? Imagine pouring your heart into groundbreaking research only to be met with skepticism. But hey, that’s exactly what happened to our boy Svante. Yet, he didn’t let that stop him. Instead, he pushed forward, proving his critics wrong and making history in the process.
Now, if you’re here because you’re curious about the Arrhenius equation or the acid-base theory, you’re in for a treat. We’re gonna break it all down for you, piece by piece, so you can truly appreciate the genius of this Swedish chemist. Plus, we’ll sprinkle in some fun facts about his personal life and other contributions to science. Let’s get started!
Read also:Exploring The Depths Of Jjda016 The Ultimate Guide
Who Was Svante Arrhenius?
Before we dive into the science, let’s take a moment to meet the man behind the theories. Svante Arrhenius was born in Sweden in 1859. He was one of those kids who just seemed to get stuff, you know? Like, he could read before he even started school. Crazy, right? His early life was filled with curiosity and a thirst for knowledge, which eventually led him to become one of the most influential chemists in history.
Early Life and Education
Arrhenius’s early life was nothing short of extraordinary. Growing up in a family that valued education, he was encouraged to explore and learn from a young age. By the time he was in his teens, he was already excelling in mathematics and physics. He attended the University of Uppsala, where he began his journey into the world of chemistry. His professors at the time probably didn’t realize they were nurturing a future Nobel Prize winner.
Key Contributions to Science
Let’s not forget why we’re really here. Arrhenius’s contributions to science are nothing short of legendary. His work on the dissociation of electrolytes and the Arrhenius equation are still taught in chemistry classes around the world. But his most famous contribution, the acid-base theory, changed the way we understand chemical reactions. It’s like he opened a whole new door for chemists everywhere.
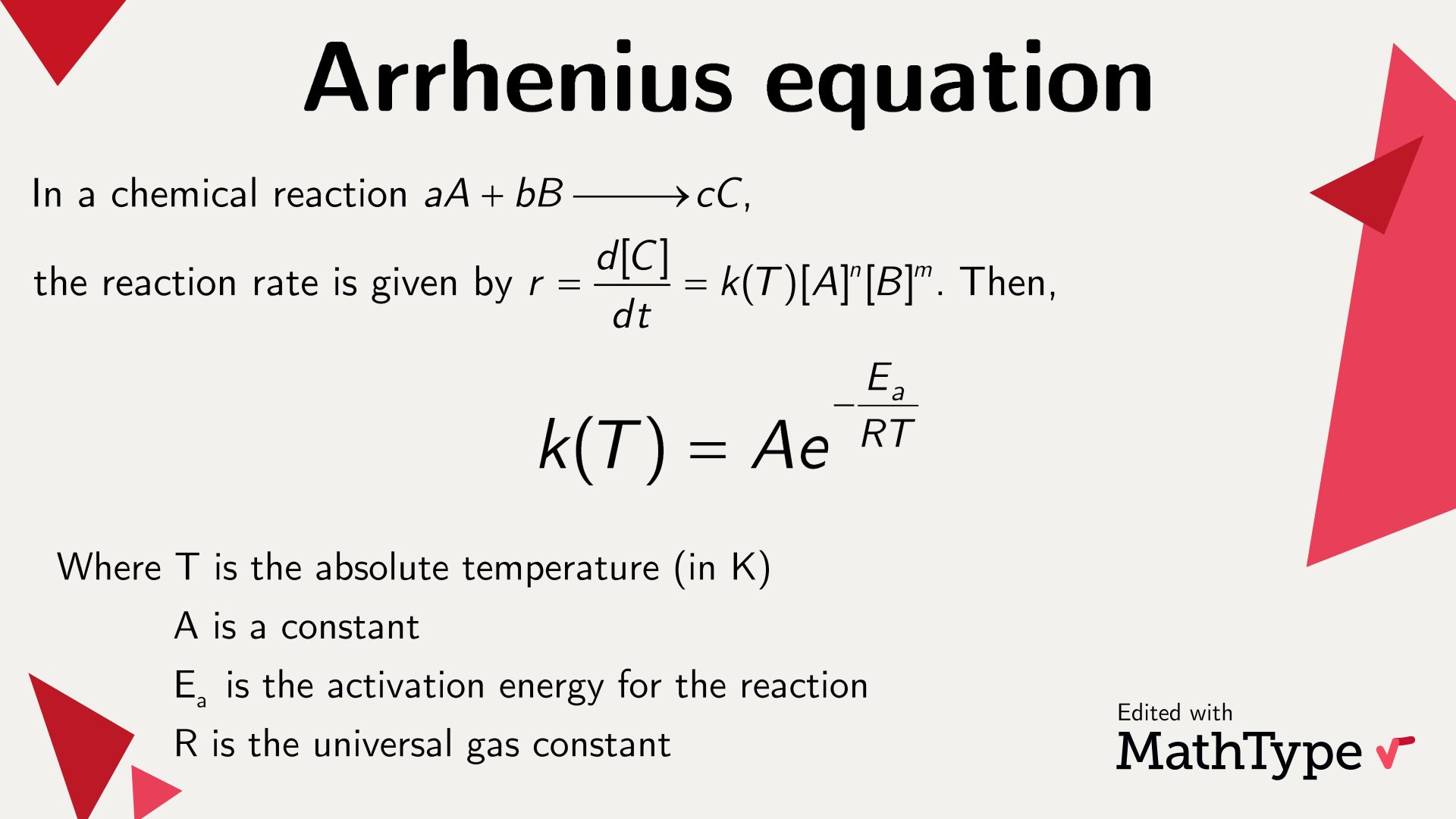
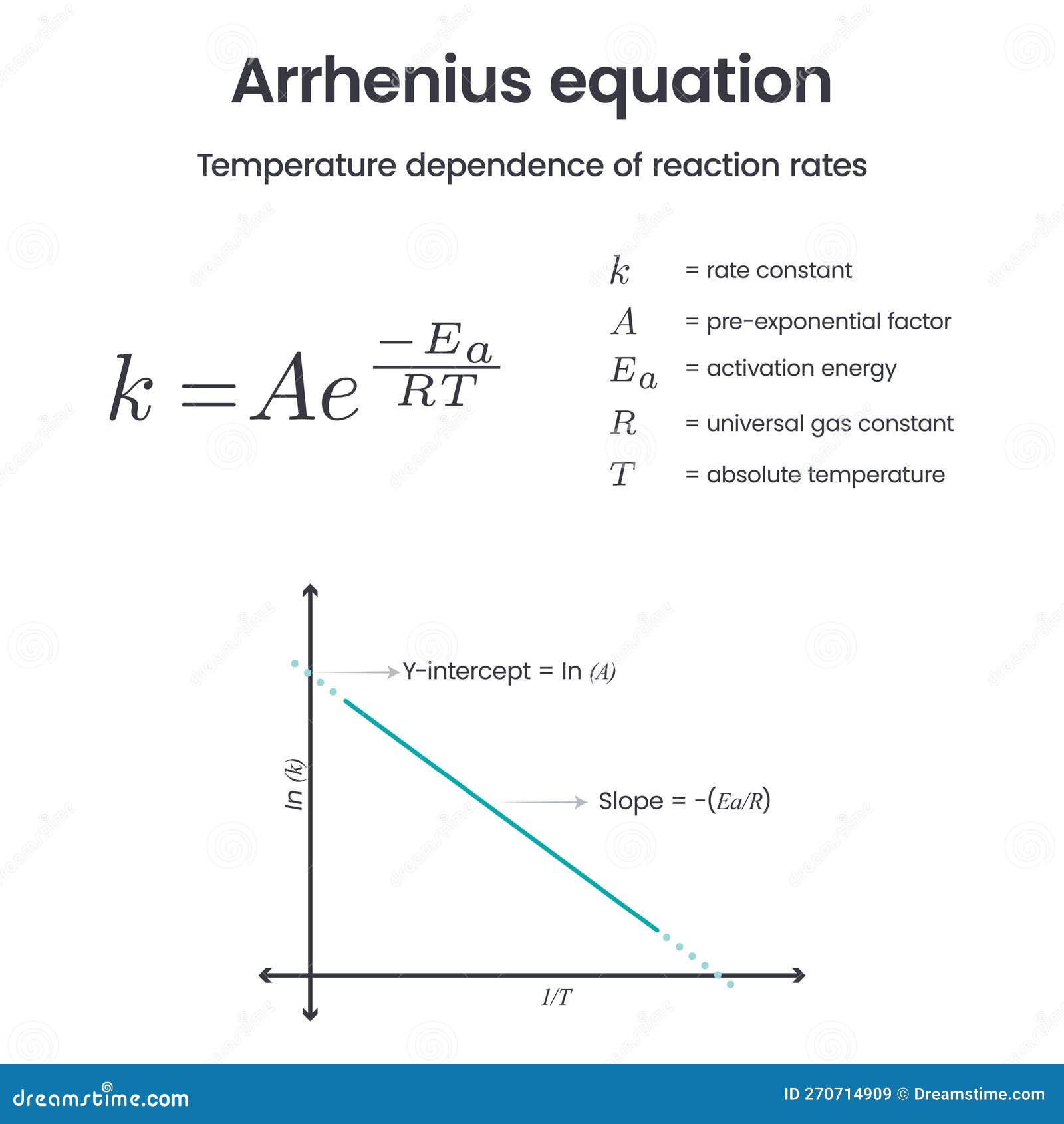
Arrhenius Equation: The Backbone of Chemical Kinetics
The Arrhenius equation is like the Swiss Army knife of chemical kinetics. It helps us understand how temperature affects the rate of a chemical reaction. In simple terms, it’s all about how fast things happen when you crank up the heat. This equation is crucial in fields ranging from industrial chemistry to environmental science. If you’ve ever wondered why your food cooks faster in a pressure cooker, you can thank Arrhenius for that insight.
Breaking Down the Arrhenius Equation
Okay, so let’s break it down. The Arrhenius equation looks something like this: k = Ae^(-Ea/RT). Don’t freak out if it looks scary; it’s actually pretty cool once you get the hang of it. ‘k’ is the rate constant, ‘A’ is the pre-exponential factor, ‘Ea’ is the activation energy, ‘R’ is the gas constant, and ‘T’ is the temperature. See? Not so bad, right?
Applications of the Arrhenius Equation
This equation isn’t just some theoretical concept; it has real-world applications. For instance, it’s used in the food industry to determine the shelf life of products. In the automotive industry, it helps in designing more efficient engines. And let’s not forget about its role in environmental science, where it’s used to model climate change and other complex processes. Arrhenius’s work is everywhere, even if you don’t realize it.
Read also:Celina Smith Riley Reid The Rising Star Shining Bright In The Entertainment World
Arrhenius Acid-Base Theory: The Foundation of Modern Chemistry
Now, let’s talk about the theory that put Arrhenius on the map. The Arrhenius acid-base theory defines acids and bases in terms of their behavior in water. Acids, according to Arrhenius, are substances that increase the concentration of hydrogen ions (H+) in water. Bases, on the other hand, increase the concentration of hydroxide ions (OH-). Simple, right? But it was revolutionary at the time.
Understanding Acids and Bases
Think about it this way. When you drink soda, you’re consuming an acid. That’s why it tastes sour. On the flip side, when you use soap, you’re using a base. It’s all about the balance, and Arrhenius helped us understand that balance. His theory laid the groundwork for future developments in chemistry, including the Brønsted-Lowry and Lewis theories.
Practical Applications of the Acid-Base Theory
The Arrhenius acid-base theory isn’t just for textbooks. It’s used in everyday life. From regulating the pH of swimming pools to developing medications, this theory is everywhere. It’s also crucial in environmental science, where it’s used to study acid rain and its effects on ecosystems. Arrhenius’s work has truly stood the test of time.
Arrhenius and the Nobel Prize
Let’s not forget the crowning achievement of Arrhenius’s career: the Nobel Prize in Chemistry. He won it in 1903 for his work on the dissociation of electrolytes. This was a big deal back then, and it cemented his place in the annals of scientific history. But here’s the thing: he didn’t win it easily. There was a lot of controversy surrounding his award, but in the end, his groundbreaking work spoke for itself.
The Controversy Surrounding the Nobel Prize
When Arrhenius won the Nobel Prize, some people in the scientific community were not happy. They thought his work was too theoretical and lacked practical applications. But as time went on, his theories proved to be invaluable. It just goes to show that sometimes, you’ve got to trust your gut and keep pushing forward, even when the world doesn’t understand you yet.
Legacy of the Nobel Prize
Winning the Nobel Prize was a game-changer for Arrhenius. It gave him the recognition he deserved and opened up new opportunities for his research. But more importantly, it inspired a whole new generation of scientists to pursue their dreams, no matter how unconventional they might seem. Arrhenius’s legacy lives on in the countless scientists who have followed in his footsteps.
Arrhenius’s Impact on Modern Science
Arrhenius’s contributions to science extend far beyond the Arrhenius equation and the acid-base theory. He was a pioneer in the field of physical chemistry, and his work has influenced countless other disciplines. From environmental science to biochemistry, his theories have had a profound impact on the way we understand the world around us.
Environmental Science
One of the lesser-known aspects of Arrhenius’s work is his contribution to environmental science. He was one of the first scientists to propose the idea of global warming, long before it became a hot-button issue. His calculations suggested that increasing levels of CO2 in the atmosphere could lead to a rise in global temperatures. Sound familiar? Yeah, Arrhenius was way ahead of his time.
Biochemistry
Arrhenius’s work also had a significant impact on the field of biochemistry. His theories about the behavior of ions in solutions helped scientists understand how enzymes work and how they catalyze chemical reactions in the body. This knowledge has been crucial in the development of modern medicine and biotechnology.
Fun Facts About Svante Arrhenius
Alright, let’s lighten things up a bit. Here are some fun facts about Svante Arrhenius that you probably didn’t know:
- He was a chess enthusiast and even played against world champion Emanuel Lasker.
- He wrote several popular science books, making complex scientific concepts accessible to the general public.
- He had a keen interest in astronomy and even proposed the idea that life on Earth might have originated from space.
Biographical Data of Svante Arrhenius
| Full Name | Svante August Arrhenius |
|---|---|
| Birth Date | February 19, 1859 |
| Place of Birth | Vik, Sweden |
| Death Date | October 2, 1927 |
| Place of Death | Stockholm, Sweden |
| Field of Work | Chemistry, Physics |
Conclusion
So there you have it, folks. Svante Arrhenius wasn’t just some guy with a cool name; he was a true scientific genius who changed the world. His contributions to chemistry, environmental science, and biochemistry have had a lasting impact on our understanding of the universe. Whether you’re a chemistry student or just someone who appreciates a good science story, Arrhenius’s life and work are definitely worth exploring.
Now, here’s the thing. If you’ve learned something from this article, why not share it with your friends? Or maybe leave a comment and let us know what you think. And hey, if you’re hungry for more science stories, stick around. We’ve got plenty more where this came from.
Table of Contents
- Who Was Svante Arrhenius?
- Arrhenius Equation: The Backbone of Chemical Kinetics
- Arrhenius Acid-Base Theory: The Foundation of Modern Chemistry
- Arrhenius and the Nobel Prize
- Arrhenius’s Impact on Modern Science
- Fun Facts About Svante Arrhenius
- Biographical Data of Svante Arrhenius
- Environmental Science
- Biochemistry
- Conclusion
/GettyImages-92831471-5c56435846e0fb00012ba77a.jpg)